International Drug Development Institute – IDDI
Your Regulatory Statistics
& Clinical Data Science Partner
Explore Our Services:
Let IDDI be the guardian of your data, turning your clinical trials into a success.
IDDI is the center of excellence in biostatistics and clinical data science with deep expertise in handling complex data challenges.
At IDDI, we are not a traditional contract research organization (CRO); we are your dedicated clinical data partner, committed to excellence from clinical trial design to regulatory submission.
We offer regulatory statistics, clinical trial design, RTSM, clinical data management, and biostatistical services for pharmaceutical, biotech, and medical device/diagnostic companies.
Therapeutic & Specialty Areas
With IDDI, drug development is powered by exceptional insights into cancer research, ophthalmology, CNS, and rare diseases combined with unmatched data quality acumen.
1,445 +
Clinical Trials
350 +
Customers Worldwide
30 +
Years of Experience
Passion + Science + Experience = IDDI
IDDI adds value to your clinical data with our:
Patient-Centric Services
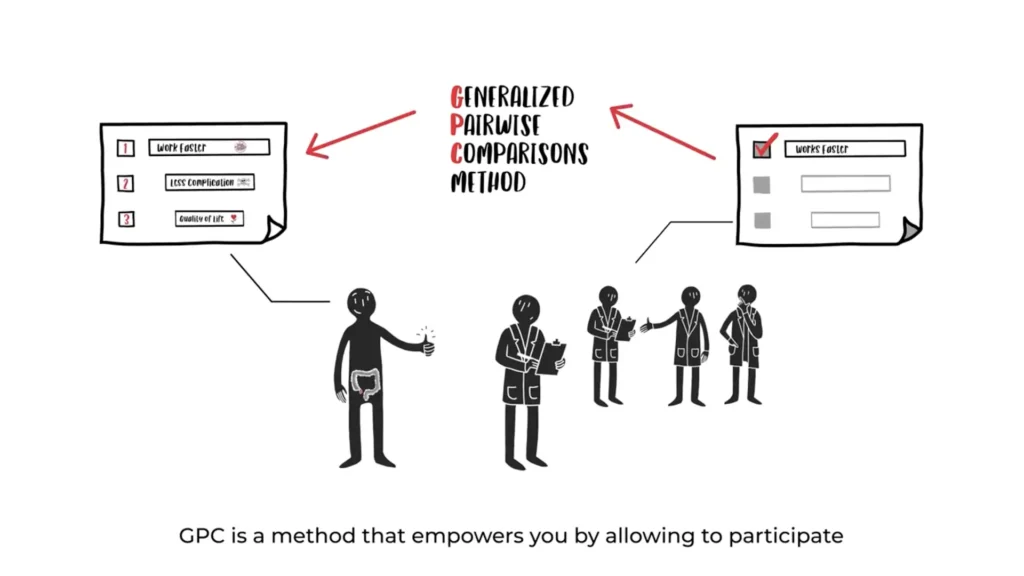
At IDDI, we are dedicated to patient-centricity, which has driven the development of our Generalized Pairwise Comparisons (GPC) method. This innovative approach empowers clinicians to provide personalized treatments that align with the unique preferences and needs of each patient. In July 2023, this research culminated in the establishment of One2Treat, a new company focused on enhancing clinical trials.
One2Treat utilizes an innovative statistical method for the design and analysis of clinical trials, concentrating on multiple dimensions to ensure that all key patient-relevant outcomes are incorporated into treatment decisions, particularly in primary analyses.
Our commitment to patient-centric services is paving the way for more effective and tailored healthcare solutions.