Electronic Data Capture
Faster, Higher-Quality Data With IDDI-Powered EDC
The success of your clinical development program depends on data quality. If you want to prioritize data quality while supporting seamless clinical trial processes, you need IDDI’s electronic data capture (EDC) solutions.
Technology + Data Excellence for Your EDC Trial
Since 2008, IDDI has implemented 159 EDC trials. Our data managers combine the scientific background, experience, and the technological expertise required to maximize the value of your clinical data.
IDDI can set up your eCRF in ID-base™ (Marvin powered by EvidentIQ) and Rave EDC (powered by Medidata). Both electronic data capture (EDC) are intuitive, cost-saving, and integrated with RTSM system.

Since 2008 IDDI has implemented:
159
EDC Studies

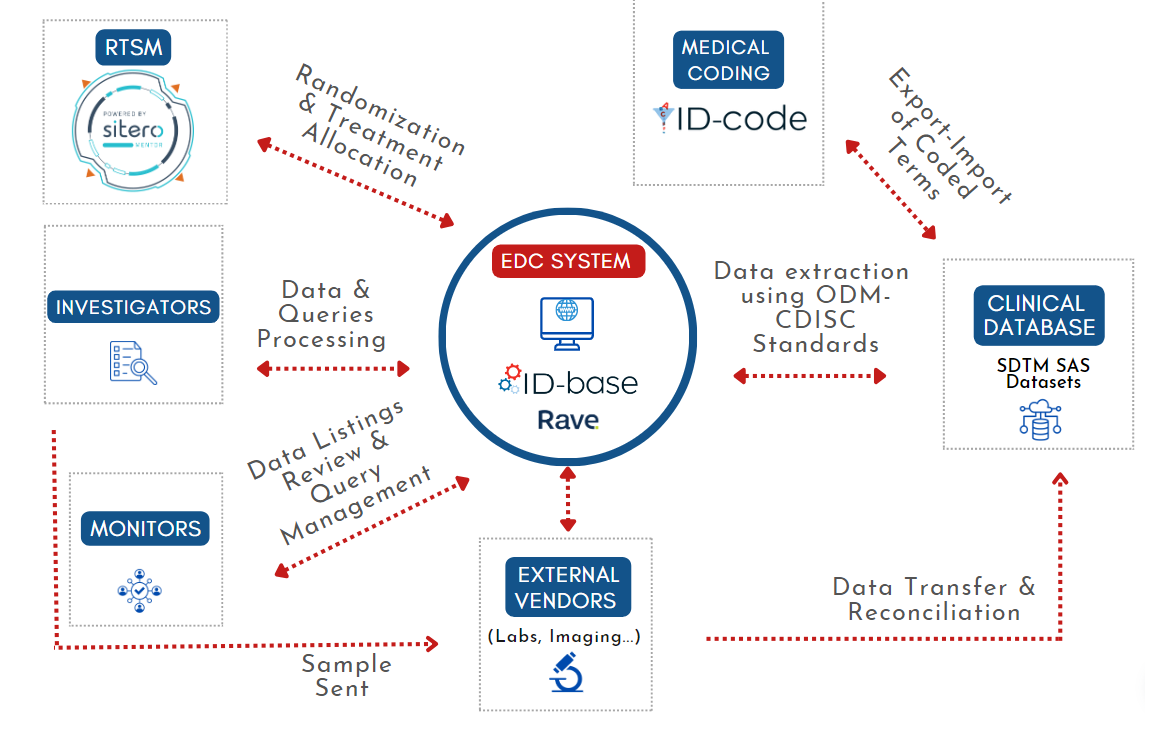

Key Features of Our EDC Systems
With features that help reduce costs and drive efficiency, our EDC solutions enable:
Ready to improve the speed and quality of your clinical trials?
Contact IDDI today to discover how our EDC solutions can transform your clinical data management strategy.