When it comes to clinical trial submission, the Analysis Data Model (ADaM) is one of the most important standards developed by the Clinical Data Interchange Standards Consortium (CDISC). ADaM provides a standardized framework for representing analysis-ready data and facilitating efficient and consistent analysis, allowing reviewers to quickly assess and approve submission.
IDDI’s ADaM – Data Standards Implementation solution leverages the latest CDISC models to generate compliant datasets from the start, ensuring adherence to the highest standards of quality and efficiency.

Driving Excellence in CDISC ADaM Compliance
As a CDISC registered provider, every IDDI study is meticulously aligned with FDA electronic submission standards, delivering database structures seamlessly transformed into ADaM formats and accompanied by the requisite documentation.
Unlike some contract research organizations (CROs) where generalist programmers are assigned a broad spectrum of tasks, our biostatisticians play a hands-on role in programming ADaM derivations and analyses, providing your research with an unmatched level of expertise and precision.
Discover ID-SPECTACKLE:
A user-friendly graphical interface making ADaM specifications “Fun”
ID-Spectackle is a user-friendly graphical interface to guide statistical programmers. This tool is meant to help and prepare the ADaM Specifications, to create Define-XML for submission. It helps to limit a lot of manual input by automating the metadata collection and provide guidance to fill-in the specifications. As the basis of the specifications, a SAS® Macro collects metadata, with support of the ADaM IG and NCI Controlled Terminology.
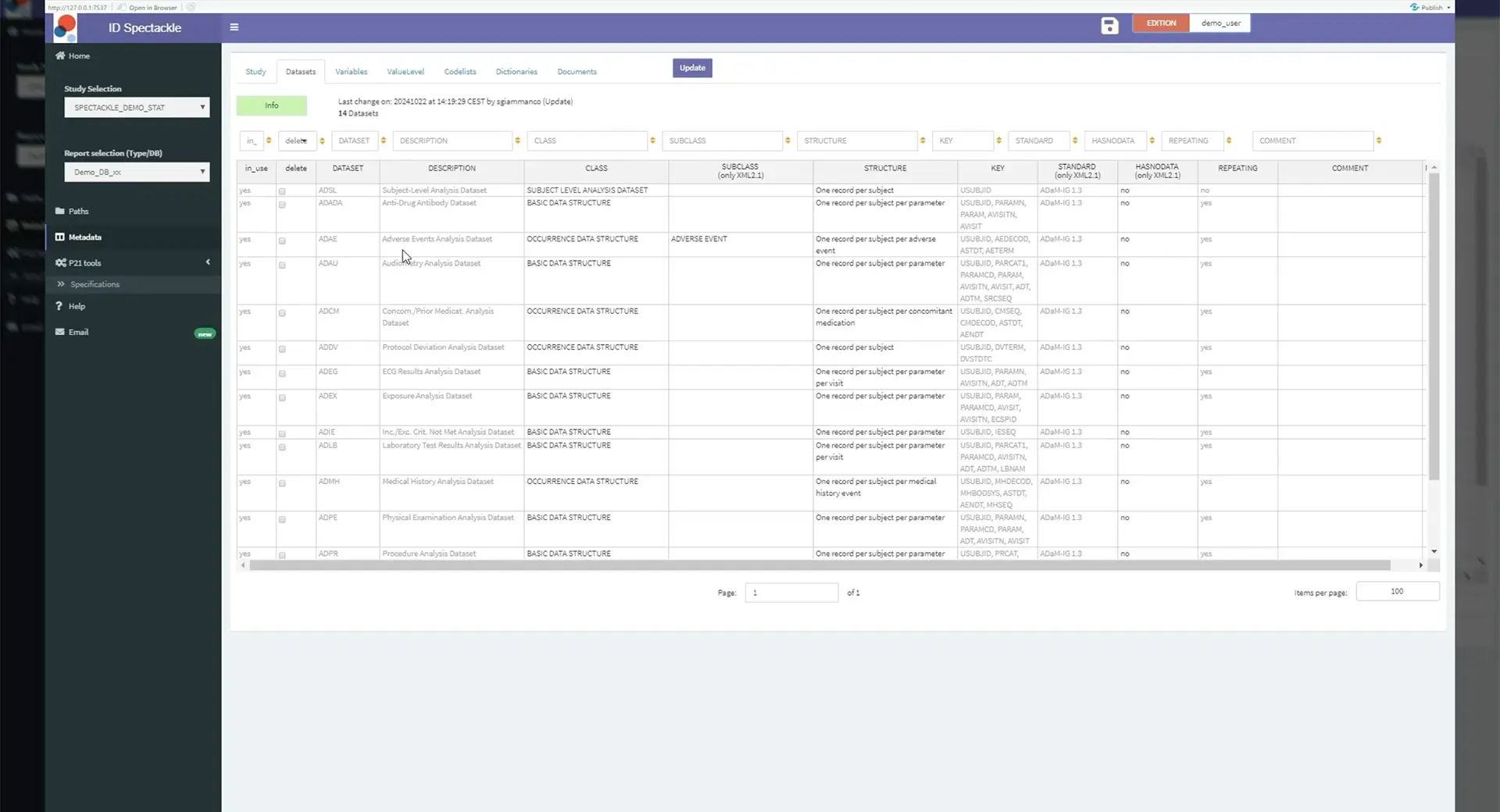
This automated process presents multiple advantages:
- Avoids error-prone copy-pasting of redundant information among sources
- Ensures that specifications and datasets are aligned
- Provides consistency checks with completion rules
- Evolution of standards can be implemented directly in the system
- Ensures that the specifications format is compliant with the one from P21, it clarifies and deploys a standard process company wide.

CDISC ADaM Services Tailored to Your Needs
Our ADaM solutions deftly navigate the complexities of CDISC compliance, making your journey toward regulatory submission easy:
- Production of ADaM datasets
- Precise creation of define.xml and define.pdf files from ADaM dataset definitions
- Electronic validation of CDISC compliance to ADaM standards
- Creation of integrated ADaM datasets for comprehensive summaries of safety & efficacy (ISS/ISE)